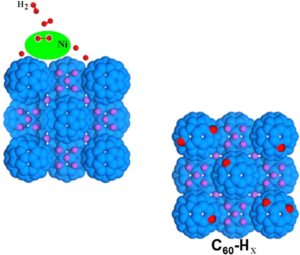
 The addition of transition metals to alkali intercalated fullerides proved to enhance their already good hydrogen absorption properties. Herein we present a study based on two different synthetic strategies, allowing the addition of nickel as aggregates with different size to the lithium fulleride Li6C60: the former is based on the metathesis of nickel chloride, while the latter on the thermal decomposition of nickel carbonyl clusters. The hydrogen-storage properties of the obtained materials have been investigated with manometric and calorimetric measurements, which indicated a clear enhancement of the final absorption value and kinetics with respect to pristine Li6C60, as a consequence of nickel surface catalytic activity towards hydrogen molecules dissociation. We found up to 10 % increase of the total H2 weight % absorbed (5.5 wt% H2) in presence of Ni aggregates. Furthermore, the control of the transition metal particles size distribution allowed reducing the hydrogen desorption enthalpy of the systems.
The addition of transition metals to alkali intercalated fullerides proved to enhance their already good hydrogen absorption properties. Herein we present a study based on two different synthetic strategies, allowing the addition of nickel as aggregates with different size to the lithium fulleride Li6C60: the former is based on the metathesis of nickel chloride, while the latter on the thermal decomposition of nickel carbonyl clusters. The hydrogen-storage properties of the obtained materials have been investigated with manometric and calorimetric measurements, which indicated a clear enhancement of the final absorption value and kinetics with respect to pristine Li6C60, as a consequence of nickel surface catalytic activity towards hydrogen molecules dissociation. We found up to 10 % increase of the total H2 weight % absorbed (5.5 wt% H2) in presence of Ni aggregates. Furthermore, the control of the transition metal particles size distribution allowed reducing the hydrogen desorption enthalpy of the systems.
Reproduced with permission. Copyright 2020, Elsevier
