We report the identification of the new intercalated fullerene polymer Mg2C60. Mg 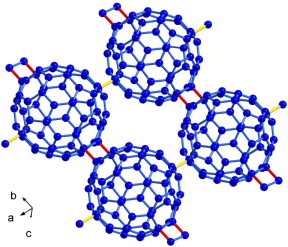 intercalation was obtained either by solid state reaction between C60 and Mg, or by thermal decomposition of the metallorganic precursor Mg–Anthracene–(THF)3. High resolution powder synchrotron and neutron diffraction data have clearly shown that Mg2C60 is isostructural to the superionic conductor Li4C60, where fullerenes form a two-dimensional network connected either by four-membered carbon rings, or single C–C bonds. Because of its peculiar structural arrangement Mg2C60 behaves as a good ionic conductor by means of uncorrelated ionic hopping across very small energy barriers (ΔE less than 100 meV), as found from DC and AC conductivity measurements, thus suggesting its possible use in future Mg-ion batteries.
intercalation was obtained either by solid state reaction between C60 and Mg, or by thermal decomposition of the metallorganic precursor Mg–Anthracene–(THF)3. High resolution powder synchrotron and neutron diffraction data have clearly shown that Mg2C60 is isostructural to the superionic conductor Li4C60, where fullerenes form a two-dimensional network connected either by four-membered carbon rings, or single C–C bonds. Because of its peculiar structural arrangement Mg2C60 behaves as a good ionic conductor by means of uncorrelated ionic hopping across very small energy barriers (ΔE less than 100 meV), as found from DC and AC conductivity measurements, thus suggesting its possible use in future Mg-ion batteries.
Reproduced with permission. Copyright 2012, Elsevier
