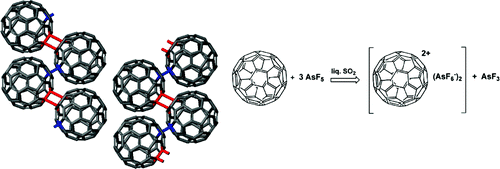
 We report on the preparation and characterization of a fullerenium salt in the solid state, where the fullerene is in the 2+ oxidized state. To succeed in this long-standing challenge, we exploit the oxidizing power of one of the strongest Lewis acids, AsF5. The weak nucleophilic character of its conjugate base is essential in stabilizing the fullerene dication in a crystal lattice. High-resolution structural analysis of this compound, with the formula C60(AsF6)2, indicates that the highly reactive C602+ units are arranged according to a novel 1D “zigzag” polymer structure. The molecules are connected by an alternating sequence of four-membered carbon rings ([2 + 2] cycloaddition) and single C−C bonds. The long awaited high-Tc superconductivity and magnetism, expected in a hole-doped C60 compound, are replaced instead by a semiconducting behavior, quite probably originating from the reduced crystal and molecular symmetry upon polymerization. The small value of the energy gap (approximately 70 meV) suggests, nevertheless, the proximity of a metallic phase.
We report on the preparation and characterization of a fullerenium salt in the solid state, where the fullerene is in the 2+ oxidized state. To succeed in this long-standing challenge, we exploit the oxidizing power of one of the strongest Lewis acids, AsF5. The weak nucleophilic character of its conjugate base is essential in stabilizing the fullerene dication in a crystal lattice. High-resolution structural analysis of this compound, with the formula C60(AsF6)2, indicates that the highly reactive C602+ units are arranged according to a novel 1D “zigzag” polymer structure. The molecules are connected by an alternating sequence of four-membered carbon rings ([2 + 2] cycloaddition) and single C−C bonds. The long awaited high-Tc superconductivity and magnetism, expected in a hole-doped C60 compound, are replaced instead by a semiconducting behavior, quite probably originating from the reduced crystal and molecular symmetry upon polymerization. The small value of the energy gap (approximately 70 meV) suggests, nevertheless, the proximity of a metallic phase.
Reproduced with permission. Copyright 2010, American Chemical Society
